Although multiple treatments exist, further research is urgently needed
Parkinson's disease (PD) is currently managed with medications that either boost dopamine levels or directly activate dopamine receptors to help control motor symptoms1-4
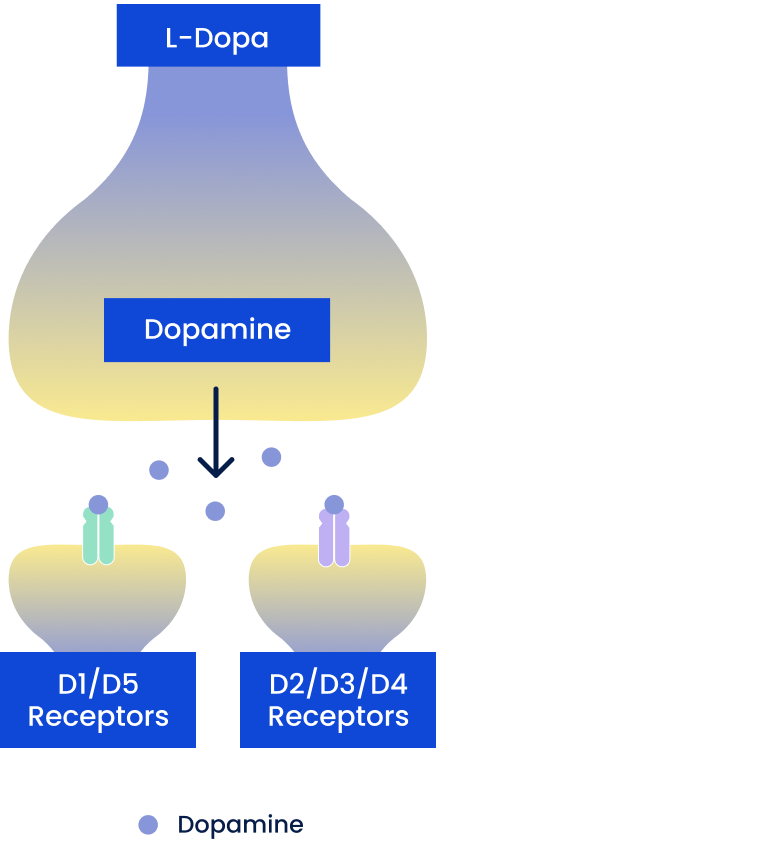
Dopamine Precursors
Include oral carbidopa/levodopa (CD/LD)
- Converted to dopamine in remaining dopaminergic neurons and released, leading to activation of all dopamine receptors
Challenges
- Over time, oral CD/LD can lose efficacy as a result of neuronal degeneration, neuronal death, and pulsatile stimulation of receptors due to its short half-life
- Patients may require more frequent and/or higher doses of oral CD/LD, which can lead to unpredictable symptom control and significant adverse effects such as dyskinesia
D2/D3/D4 Dopamine Agonists
Include pramipexole, ropinirole, rotigotine, and apomorphine†
- Mimic endogenous dopamine and act on dopamine receptors
Challenges
- D2/D3/D4 agonists activate receptors that are located throughout many parts of the brain, including areas outside of the nigrostriatal (motor) pathway
- This wide activation of D2, D3, and D4 receptors is associated with potential burdensome nonmotor adverse effects (eg, impulse control disorders, somnolence, hallucinations, edema)
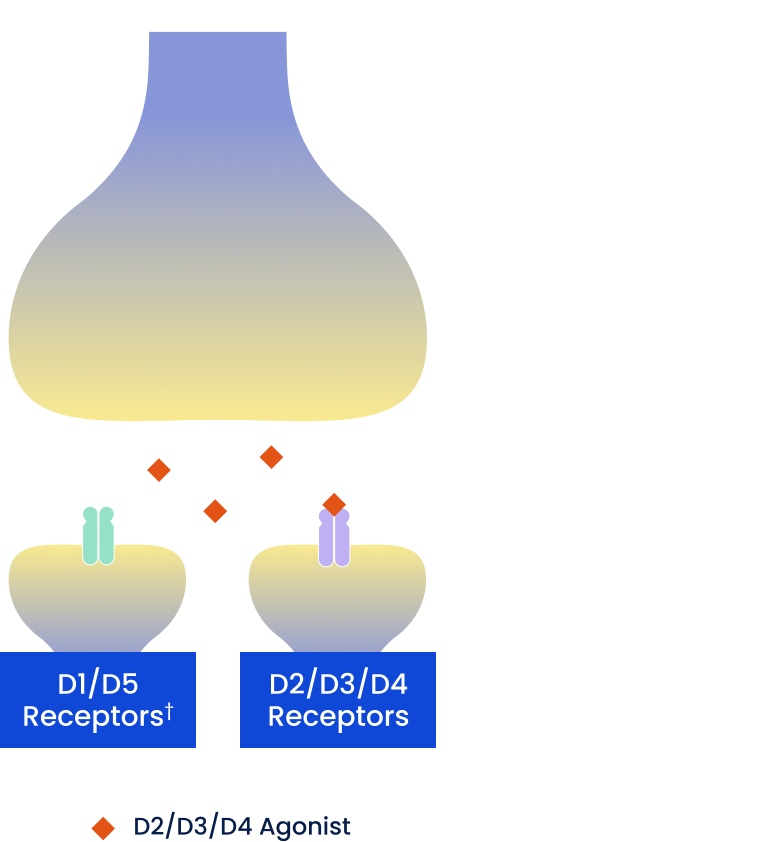
†Some D2/D3/D4 dopamine agonists may also have low/moderate binding affinity to D1-like receptors.
Dopamine Enhancers
Include MAO-B inhibitors: selegiline, rasagiline; COMT inhibitors: entacapone, opicapone, tolcapone
- Increase synaptic dopamine by decreasing enzymatic degradation
Challenges
- May only be used as adjunct therapies* (eg, in combination with oral CD/LD)
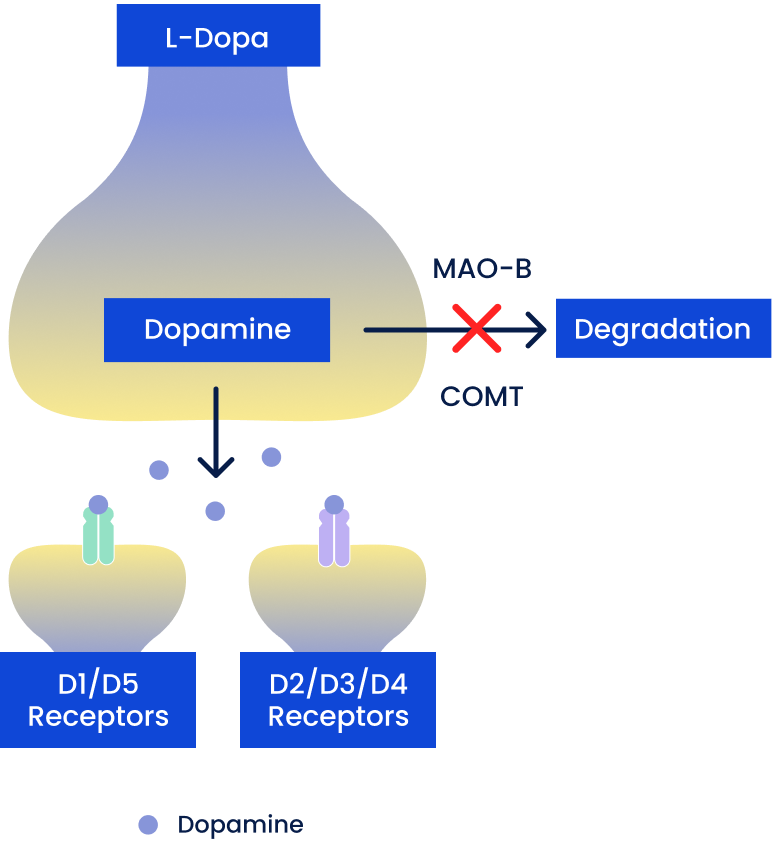
*Except for MAO-B inhibitors (namely, rasagiline), which are approved for monotherapy.
Adverse effects associated with current oral PD treatments may contribute to emotional and physical burden1,4,5
of patients develop levodopa-induced dyskinesia after 5 years of treatment with oral levodopa6
of US patients treated with current D2/D3/D4 agonists had to change therapy due to adverse events7
A closer look at the motor and nonmotor adverse effects that are associated with currently approved D2/D3/D4 agonists4,8-12

Motor fluctuations and dyskinesia

Somnolence

Dementia and psychosis

Depression

Dizziness

Orthostatic hypotension

Edema
Research into therapeutic approaches that reduce the adverse effects seen with current D2/D3/D4 agonists is ongoing
Discover emerging research on D1/D5 receptor selectivity
COMT=catechol-O-methyltransferase; MAO-B=monoamine oxidase-B.
References: 1. Poewe W, Mahlknecht P. Pharmacologic treatment of motor symptoms associated with Parkinson disease. Neurol Clin. 2020;38(2):255-267. doi:10.1016/j.ncl.2019.12.002 2. Ellis JM, Fell MJ. Current approaches to the treatment of Parkinson’s disease. Bioorg Med Chem Lett. 2017;27(18):4247-4255. doi:10.1016/j.bmcl.2017.07.075 3. Cacabelos R. Parkinson's disease: from pathogenesis to pharmacogenomics. Int J Mol Sci. 2017;18(3):551. doi:10.3390/ijms18030551 4. Isaacson SH, Hauser RA, Pahwa R, Gray D, Duvvuri S. Dopamine agonists in Parkinson's disease: impact of D1-like or D2-like dopamine receptor subtype selectivity and avenues for future treatment. Clin Park Relat Disord. 2023;9:100212. doi:10.1016/j.prdoa.2023.100212 5. Váradi C. Clinical features of Parkinson's disease: the evolution of critical symptoms. Biology (Basel). 2020;9(5):103. doi:10.3390/biology9050103 6. Grandas F, Galiano ML, Tabernero C. Risk factors for levodopa-induced dyskinesias in Parkinson’s disease. J Neurol. 1999;246(12):1127-1133. doi:10.1007/s004150050530 7. Navaratnam P, Arcona S, Friedman HS, Leoni M, Shaik S, Sasane R. Natural history and patterns of treatment change in Parkinson’s disease: a retrospective chart review. Clin Park Relat Disord. 2022;6:100125. doi:10.1016/j.prdoa.2021.100125 8. Grall-Bronnec M, Victorri-Vigneau C, Donnio Y, et al. Dopamine agonists and impulse control disorders: a complex association. Drug Saf. 2018;41(1):19-75. doi:10.1007/s40264-017-0590-6 9. Borovac JA. Side effects of a dopamine agonist therapy for Parkinson’s disease: a mini-review of clinical pharmacology. Yale J Biol Med. 2016:89(1):37-47. 10. Napier TC, Persons AL. Pharmacological insights into impulsive-compulsive spectrum disorders associated with dopaminergic therapy. Eur J Neurosci. 2019;50(3):2492-2502. doi:10.1111/ejn.14177 11. Powell A, Ireland C, Lewis SJG. Visual hallucinations and the role of medications in Parkinson's disease: triggers, pathophysiology, and management. J Neuropsychiatry Clin Neurosci. 2020;32(4):334-343. doi:10.1176/appi.neuropsych.19110316 12. de Bie RMA, Clarke CE, Espay AJ, Fox SH, Lang AE. Initiation of pharmacological therapy in Parkinson's disease: when, why, and how. Lancet Neurol. 2020;19(5):452-461. doi:10.1016/S1474-4422(20)30036-3
